Don't wait
Act now!
Use the time until your next check-up to improve your vaginal health with DeflaGyn® gel.
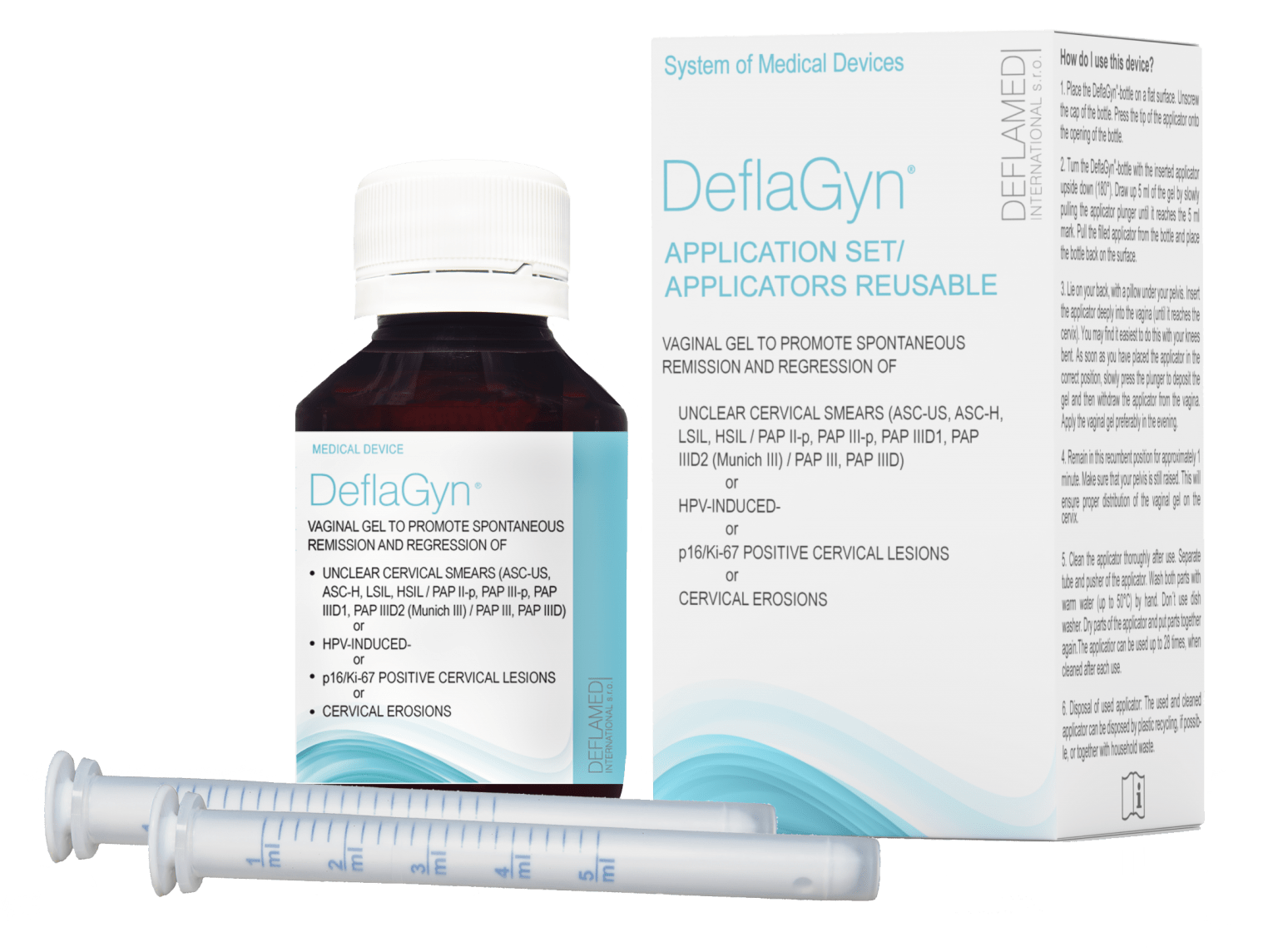
Act now with DeflaGyn® vaginal gel
DeflaGyn® helps in remission and spontaneous regression of HPV-induced cervical cell changes, or uncertain findings such as ASC-US, p16/Ki-67 positive, or even cervical erosions.
Take action like many women around the world. Don't delay anymore – act now!

DeflaGyn® Application Set
The vaginal gel is available as part of the DeflaGyn® Application Set, a medical device system that includes the gel in a bottle and applicators for convenient dosing and administration.
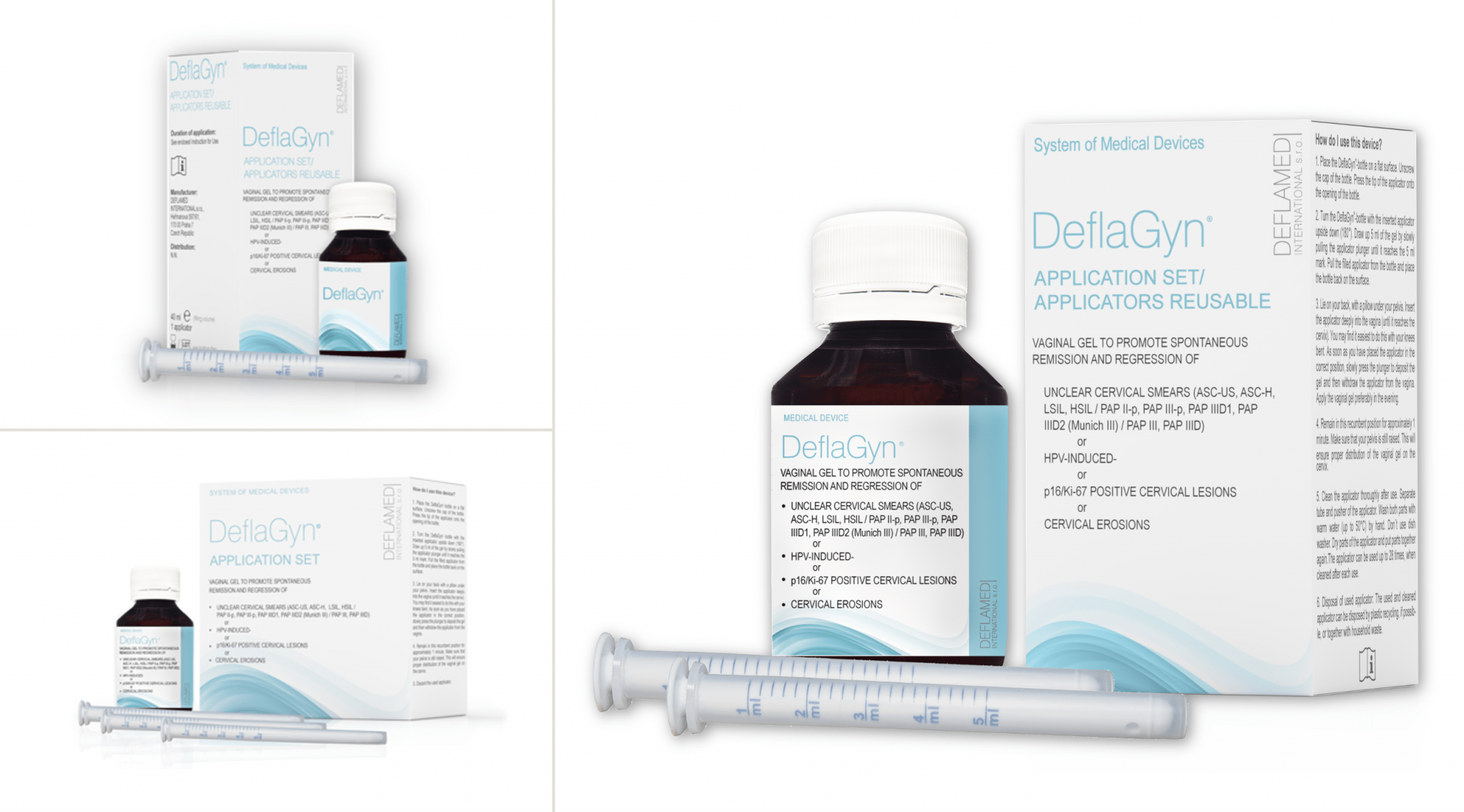
Available packs
- DeflaGyn® 150ml / 2 reusable applicators – 28 days therapy / one cycle
- DeflaGyn® 40ml / 1 reusable applicator – 7 days therapy (travel pack)
- DeflaGyn® 150ml / 28 single-use applicators – 28 days therapy / one cycle (to be discontinued)
Active Ingredients
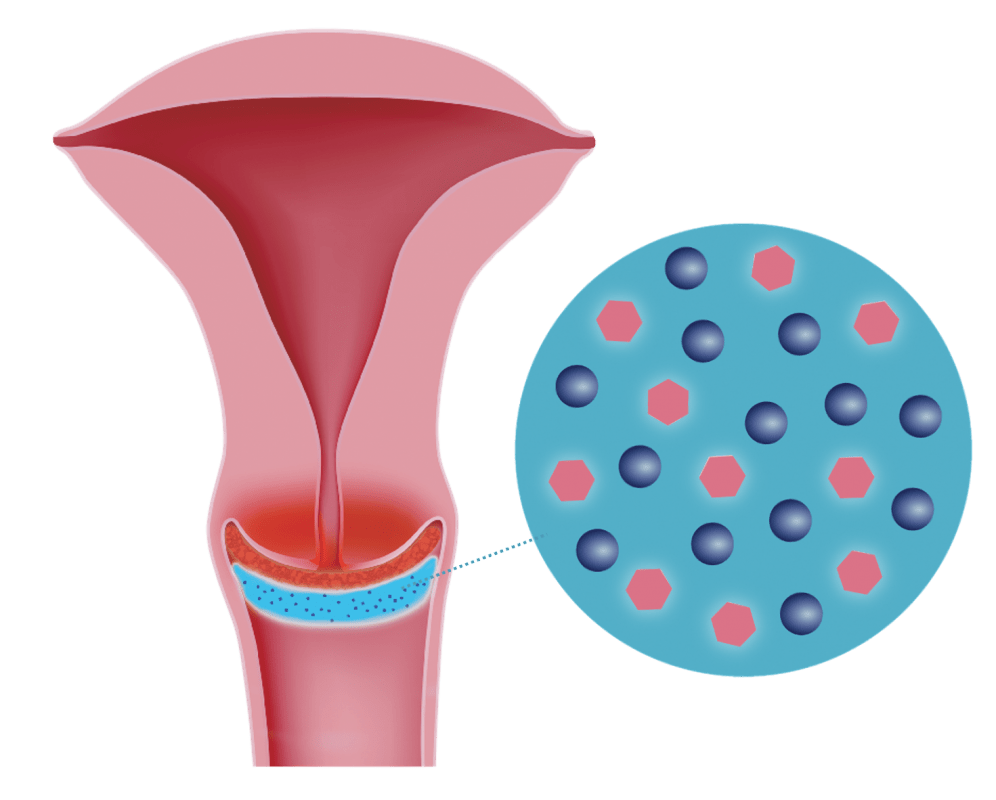
DeflaGyn® vaginal gel contains fine particles of Silicon Dioxide (SiO2) as the main component and DEFLAMIN® as an antioxidant (patented combination with Sodium Selenite and Citric Acid).
Composition of DeflaGyn® vaginal gel
| Ingredients | Amount1 (mg/5ml) | Function |
|---|---|---|
| Active ingredients | ||
| Colloidal anhydrous silica (silicon dioxide) | 10.0 | Inert adsorbent |
| DEFLAMIN® Citric acid |
24.8 | pH regulator and antioxidant enhancer |
| Sodium selenite pentahydrate2 | 0.83 | Antioxidant |
| Excipients | ||
| Water | 4899.7 | Solvent |
| Hydroxyethyl cellulose | 99.24 | Gelling agent |
| Preservatives | ||
| Potassium sorbate | 5.0 | Preservative |
| Sodium benzoate | 2.5 | Preservative |
1 Single application
2 Selenium equivalent: 0.25 mg
Three-step mechanism of action
1. Adsorption Effect
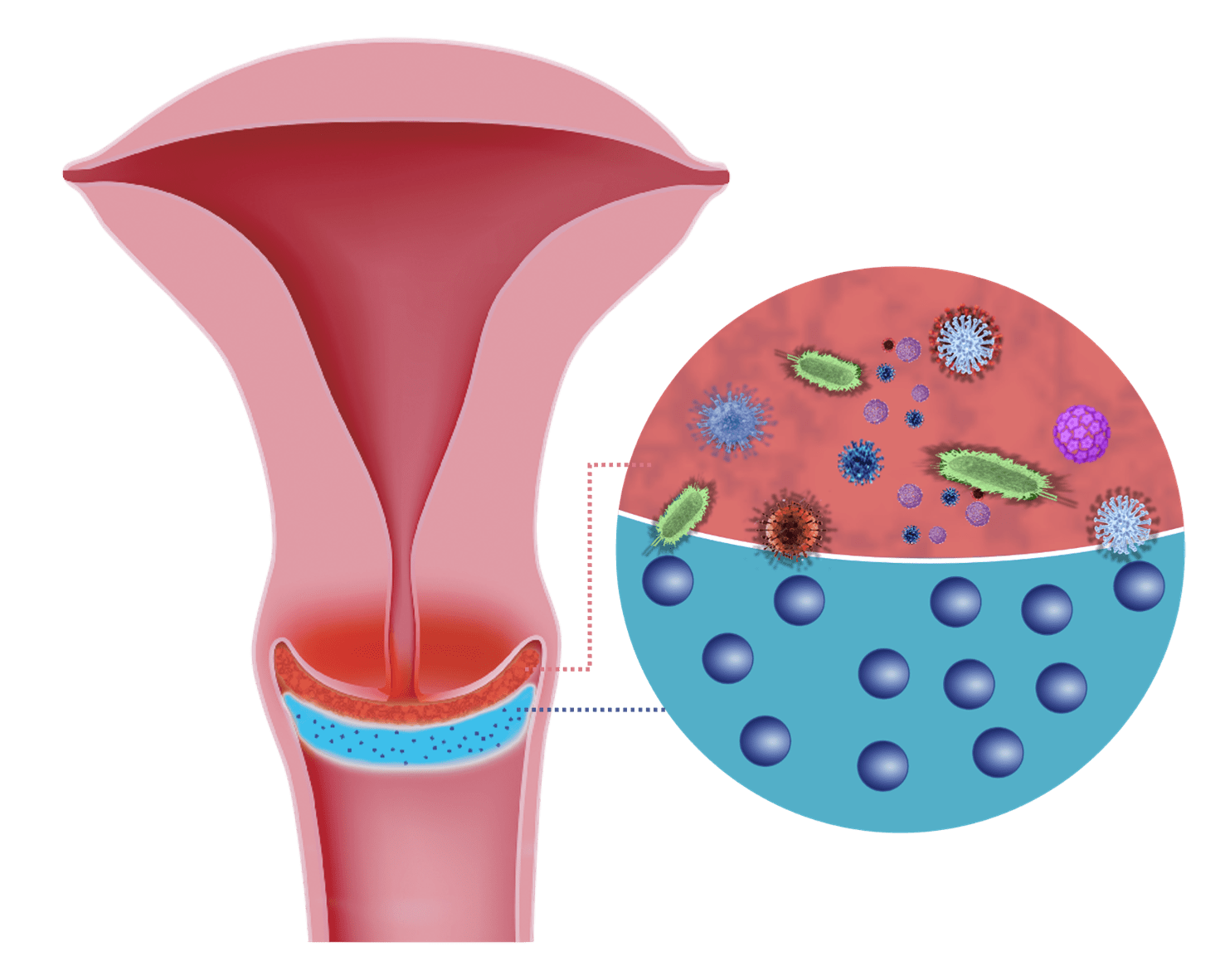
Fine particles of Silicon Dioxide adsorb pathogens (bacteria, viruses, fungi, cellular debris, irritant particles) from the cervical surface.
The "adsorbing" effect of micronized silicon has been verified and documented through fluorescence microscopy by Zeta.
Tests were performed by Partikel Analytik GmbH with Staphylococcus aureus and are fully documented.
2. Binding Effect
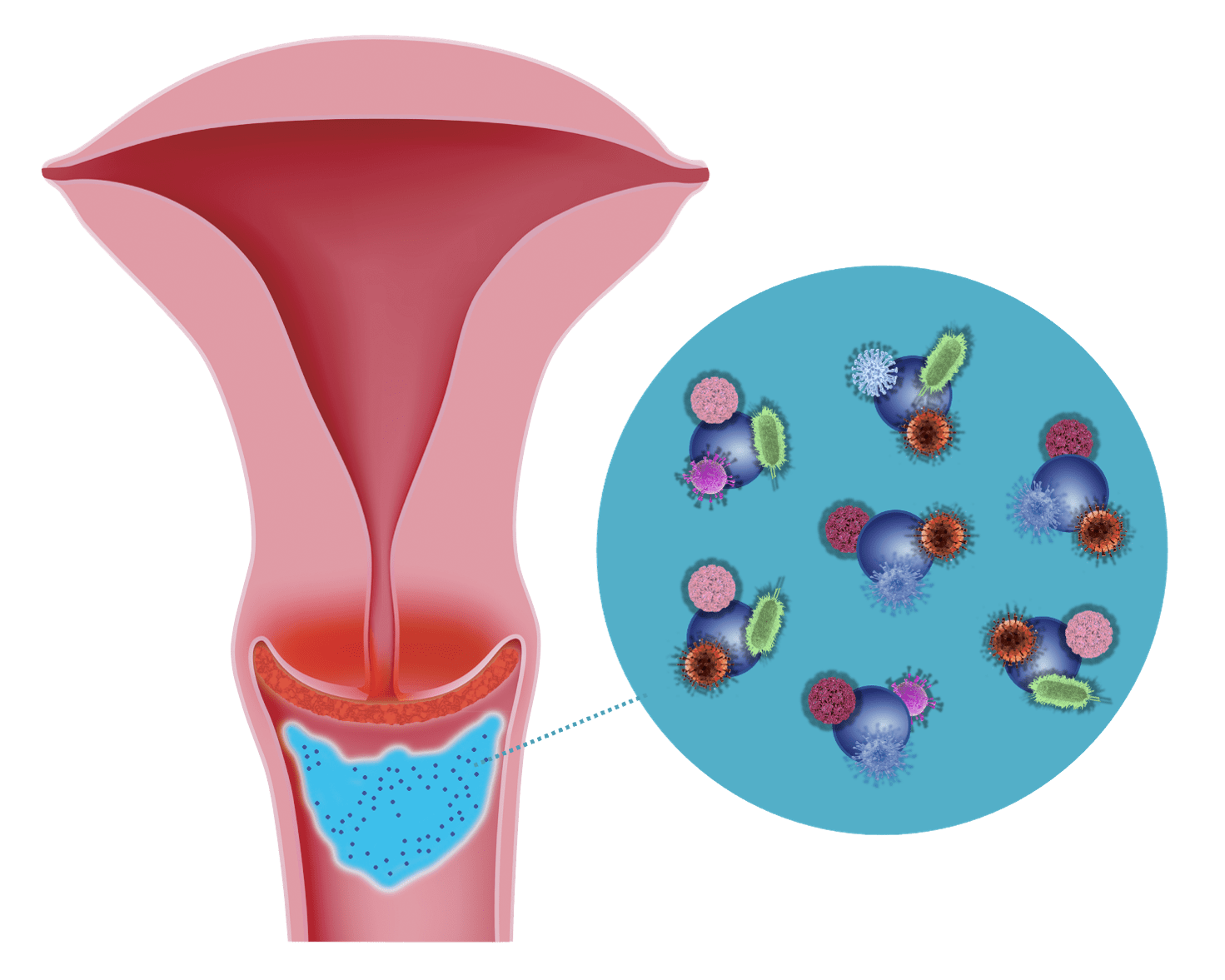
Silicon dioxide binds these pathogens and thus prevents their spread on the cervical surface and in the vagina.
The "binding effect" of micronized silicon dioxide has been verified and quantified through in-vitro tests using a vaginal secretion model system by Prof. Geoffrey Lee / Friedrich-Alexander University of Erlangen.
The tests, performed with Bovine Serum Albumin (BSA), are described in detail.
3. Antioxidative Effect
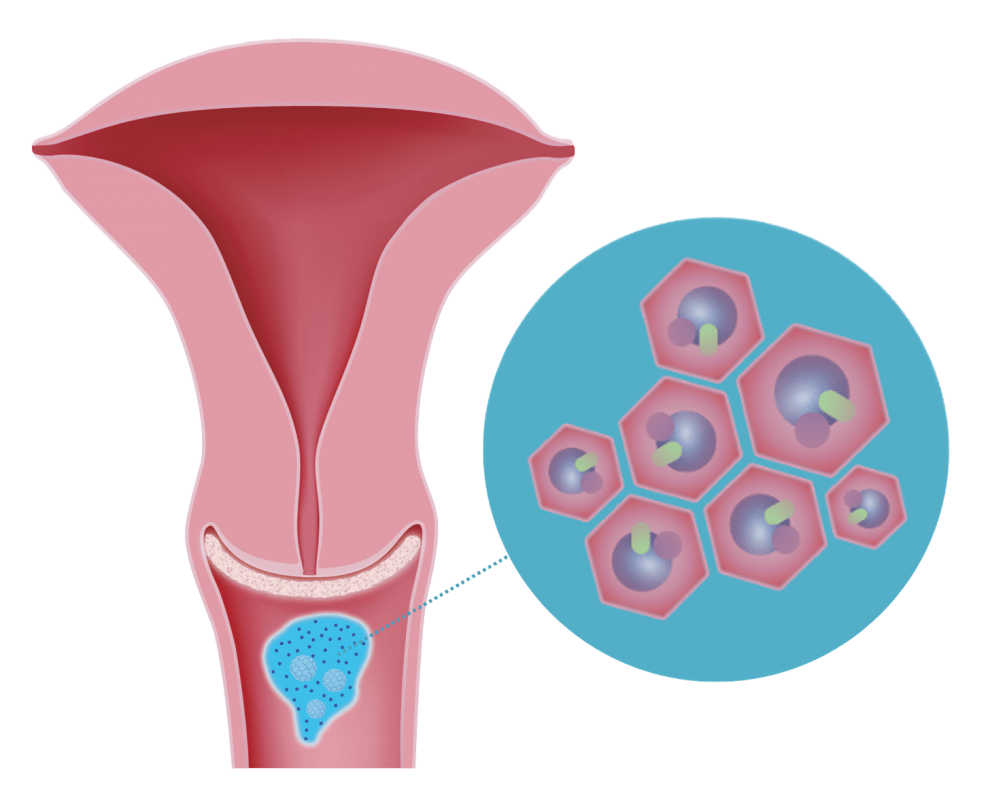
Bound pathogens are neutralized by the antioxidative properties of DEFLAMIN®.
Once bound, the potentially pro-oxidant effect of adsorbed pathogens is neutralized by DEFLAMIN®.
Once bound, the pro-oxidant effect of adsorbed pathogens is effectively neutralized by the protective properties of the formula.
Antioxidative Capacity
The antioxidative capacity of DEFLAMIN® (combination of citric acid and sodium selenite) is higher than that of the individual components and even stronger than that of vitamin C.
Furthermore, citric acid lowers and stabilizes the pH level in the vaginal environment. This is a beneficial side effect – because pH reduction alone would not be sufficient to combat pathogens, due to acid-resistant bacteria and viruses, especially the HPV virus.
Oxygen Radical Absorbance Capacity (ORAC): When measuring, vitamin E derivative (Trolox) is used as reference – the result is given in Trolox equivalents (TE).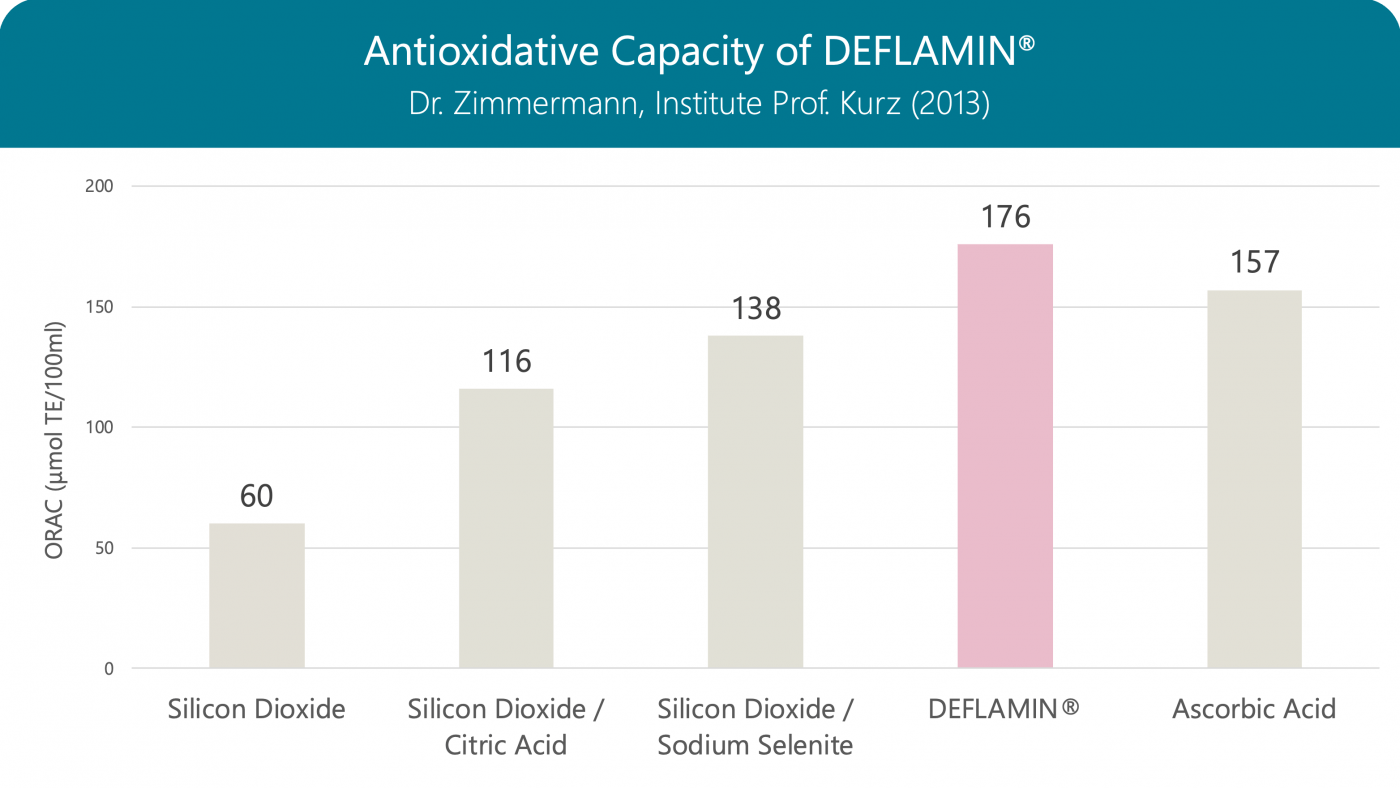
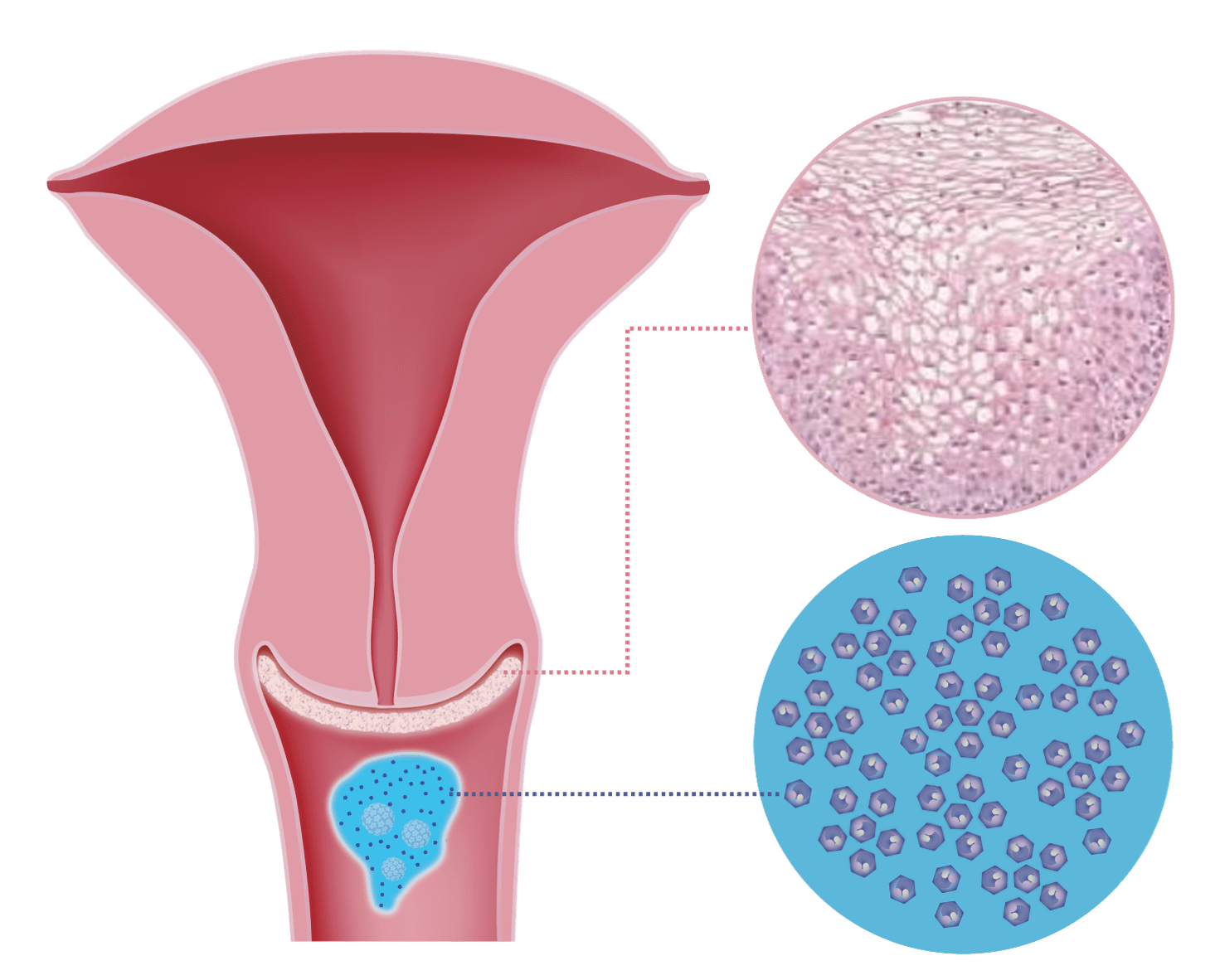
Pap test and Human Papillomavirus (HPV)

PAP test result can be normal, unclear or abnormal. If results are unclear, further observation or other measures may be required to determine the risk.
Pap Test Results


Waiting periods are common when results are unclear. This allows the body to heal itself, while ensuring careful monitoring of any possible changes.
Watchful Waiting


Waiting periods are common when results are unclear. This allows the body to heal itself, while ensuring careful monitoring of any possible changes.
How it works
Thanks to the three-step mechanism described above, the irritated cervical epithelium calms down, improving conditions for spontaneous recovery.
Adsorbed, bound and neutralized pathogens are eliminated through natural discharge of the vaginal gel.
Why it is so effective
According to guidelines for the "watchful waiting" period, using DeflaGyn® vaginal gel for a period of 3 x 28 days helps support the natural regeneration of the cervical mucosa.
The gel soothes the irritated epithelium and vaginal mucosa from potentially irritating pathogens.
It creates a favorable environment for spontaneous regeneration during a psychologically stressful period, such as "watchful waiting".

Improved cytological results
Cytology after 3 months
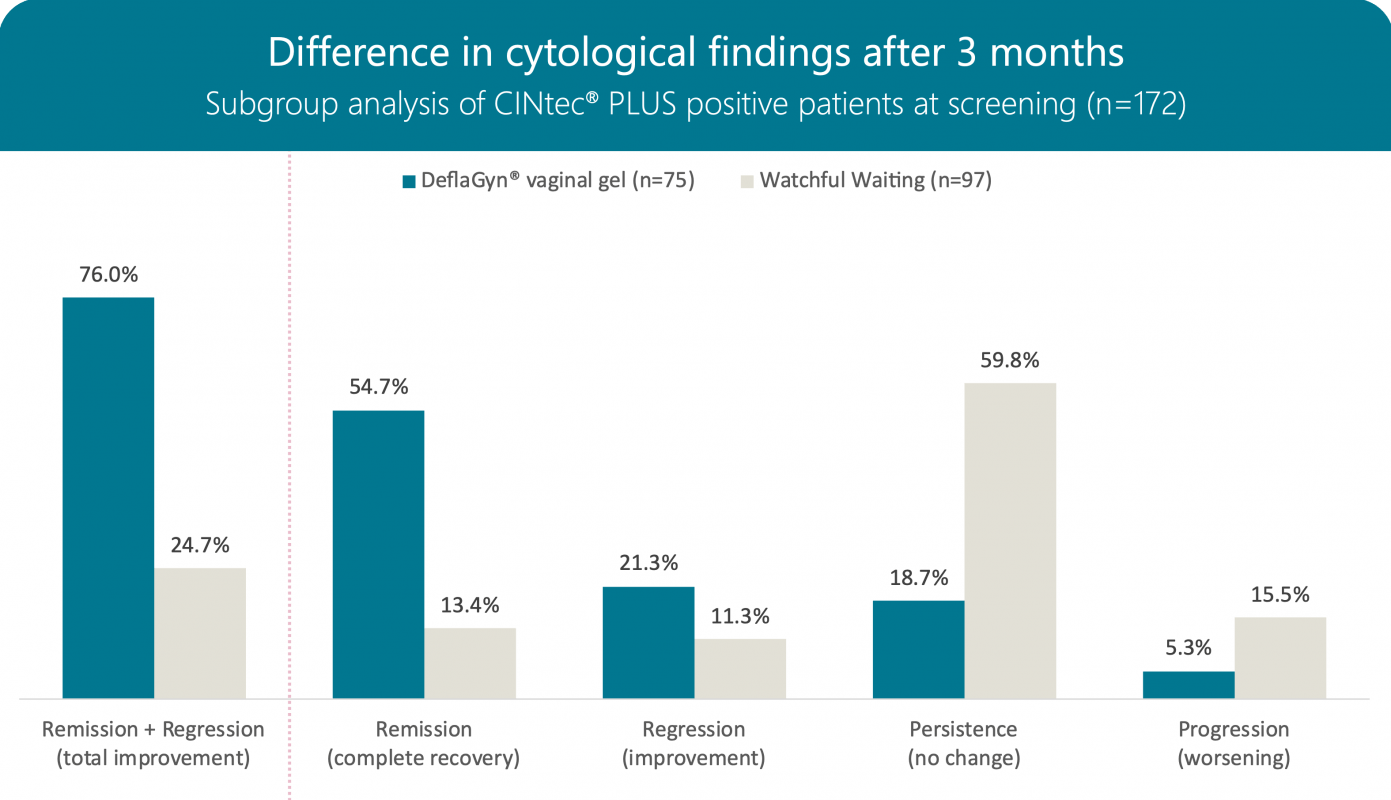
172 women with a histological diagnosis of CIN2 or p16-positive CIN1 lesions were selected based on a positive p16/Ki-67 cytological test.
For 3 months, 75 patients in the active group received 5ml of DeflaGyn® vaginal gel daily. 97 patients in the control group received no treatment ("watchful waiting").
After 3 months, cytological remission and regression were observed in 76% (57/75) of patients in the active group compared to 25% (24/97) in the control group. Progression occurred in only 5% (4/75) of the active group versus 15% (15/97) of the control group.
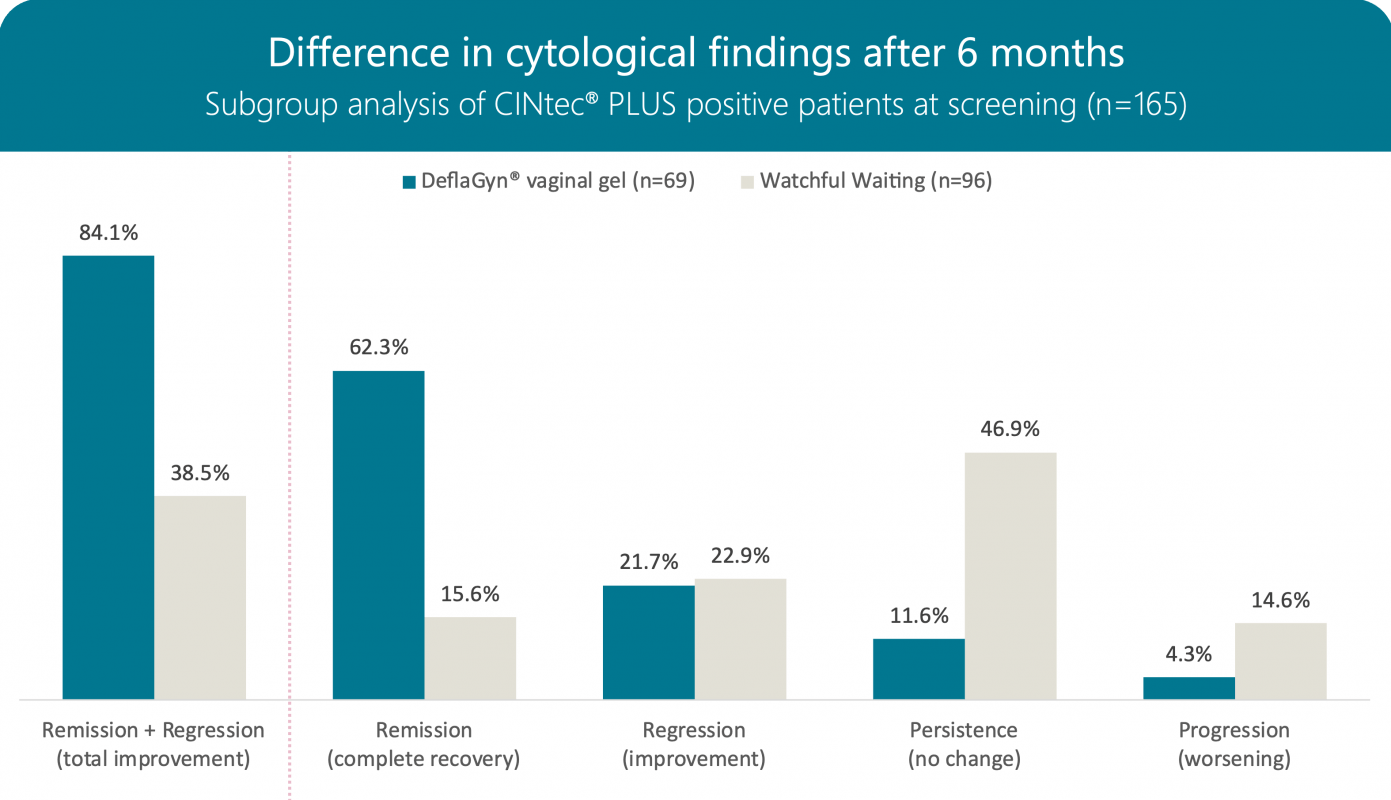
Cytology after 6 months
After a period of observation without treatment in the active group, a statistically significant change was also observed after 6 months, mainly due to the disappearance of low-grade cytological changes (ASC-US and LSIL).
58 out of 69 patients (84%) in the active group and 37 out of 96 (39%) in the control group showed regression or recovery.
DeflaGyn® is effective in promoting regression and recovery of cervical lesions and preventing their progression.
Major AL, etj. (2021) An Adsorptive and Antioxidant Vaginal Gel Clears High-Risk HPV... doi: 10.3389/fmed.2021.645559
HPV is the most common viral infection of the reproductive tract. Most sexually active women and men will be infected at some point in their lives, and some may be repeatedly infected. More than 90% of the infected population clears the infection on its own.
Although most HPV infections clear on their own and most precancerous lesions disappear spontaneously, there is a risk for all women that the infection will become chronic and precancerous lesions will progress to cervical cancer.
It usually takes 15 to 20 years for cervical cancer to develop in women with a normal immune system. In women with a weakened immune system (e.g., with untreated HIV), it can happen in just 5 to 10 years (WHO).
Effect of DeflaGyn® vaginal gel on high-risk HPV
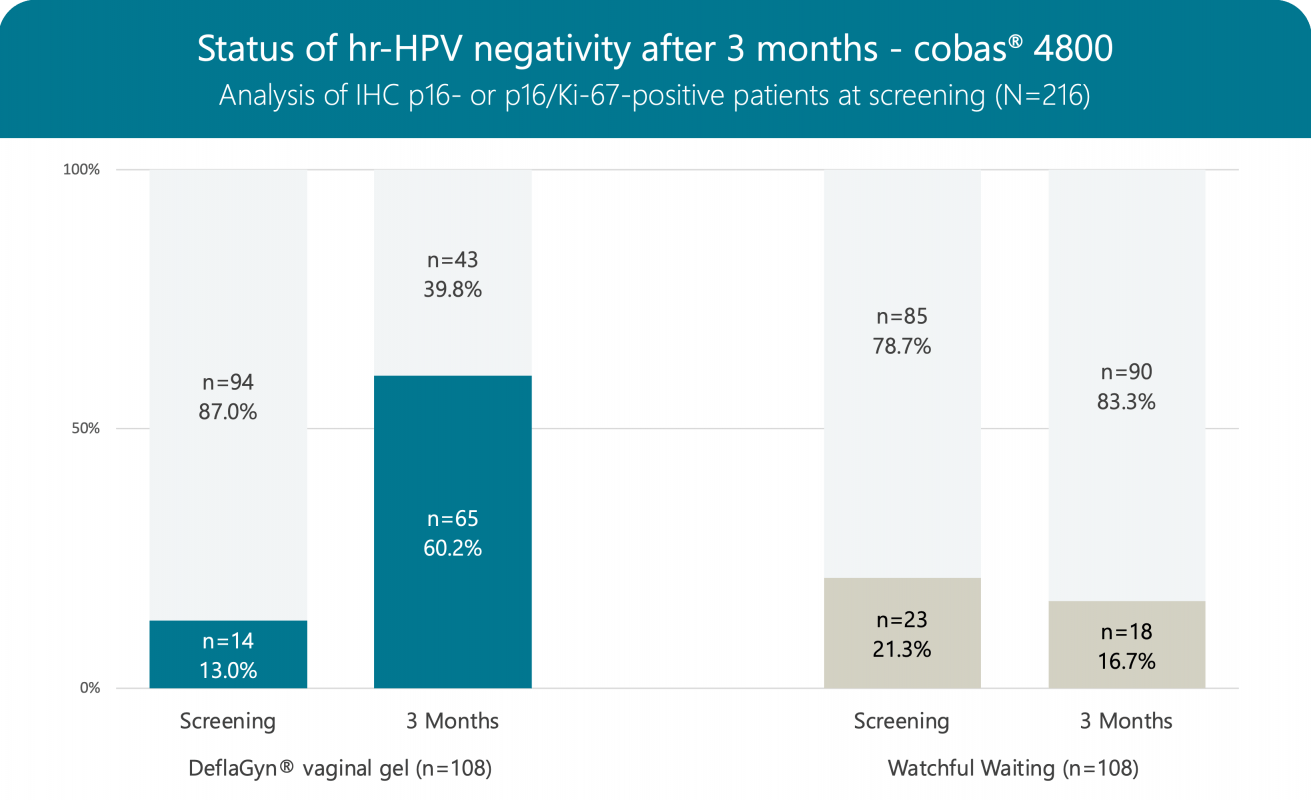
Clearance of high-risk HPV (positive at baseline vs. negative after 3 months) is 54.3% in patients treated with DeflaGyn® vaginal gel, compared to only 10.6% in patients who did not receive treatment, following the "watchful waiting" strategy.
Of 14 patients who were negative for hr-HPV at baseline, all remained negative after 3 months in the treated group, while of 23 patients in the untreated group, 14 (60.9%) became positive after 3 months.
DeflaGyn® vaginal gel binds, neutralizes and eliminates high-risk HPV, supporting the recovery of cervical and vaginal epithelium and physiological regeneration.
DeflaGyn® is a potential treatment for patients with HPV-infected cervical lesions.
High-risk HPV after 3 months
| High-risk HPV status at baseline | Patients n (%) | HPV status after 3 months | |
|---|---|---|---|
| Positive | Negative | ||
| DeflaGyn® vaginal gel – Active Group1 | |||
| Positive | 94 (87.0) | 43 (45.7) | 51 (54.3) |
| Negative | 14 (13.0) | 0 (0.0) | 14 (100.0) |
| Total | 108 (100.0) | 43 (39.8) | 65 (60.2) |
| Watchful Waiting – Control Group2 | |||
| Positive | 85 (78.7) | 76 (89.4) | 9 (10.6) |
| Negative | 23 (21.3) | 14 (60.9) | 9 (39.1) |
| Total | 108 (100.0) | 90 (83.3) | 18 (16.7) |
1 Active group: 3 months treatment with DeflaGyn® vaginal gel
2 Control group: 3 months "watchful waiting" without treatment
The CINtec® PLUS cytological test simultaneously detects the presence of two biomarkers p16 and Ki-67 (dual-stain). This abnormality is associated with high-risk HPV infections that have oncogenic activity and, if left untreated, may progress to cancer.
The molecular mechanism to detect p16 overexpression is independent of HPV type. A positive result of these biomarkers in a single cell indicates high risk for the development of cervical cancer.
Women with negative results on the dual-stain test have a lower risk for cervical disease and their body may have more time to clear the HPV infection naturally. This helps reduce frequent medical check-ups, saving time and stress for patients.
Effect of DeflaGyn® vaginal gel on HPV infections with oncogenic activity
DeflaGyn® vaginal gel adsorbs, binds, neutralizes and eliminates infectious particles from vaginal secretions and cervical mucosa. This biophysical mechanism of action enables physiological regeneration of the epithelium and supports spontaneous remission.
With daily use for 3 months, the recurrence of HPV infection in the epithelium is minimized and in many cases completely stops.
DeflaGyn® may change the oncogenic activity of high-risk HPV by improving the vaginal environment within three months of treatment.
p16/Ki-67 after 3 months treatment with DeflaGyn® vaginal gel
| CINtec® PLUS status at baseline | Patients n (%) | CINtec® PLUS status after 3 months | ||
|---|---|---|---|---|
| No data | Negative | Positive | ||
| DeflaGyn® vaginal gel – Active Group1 | ||||
| Negative | 31 (28.7) | 1 (3.2) | 30 (96.8) | 0 (0.0) |
| Positive | 77 (71.3) | 5 (6.5) | 59 (76.6) | 13 (16.9) |
| Missing | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 108 (100.0) | 6 (5.6) | 89 (82.4) | 13 (12.0) |
| "Watchful Waiting" – Control Group2 | ||||
| Negative | 8 (7.4) | 0 (0.0) | 6 (75.0) | 2 (25.0) |
| Positive | 99 (91.7) | 1 (1.0) | 20 (20.2) | 78 (78.8) |
| Missing | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Total | 108 (100.0) | 1 (0.9) | 26 (24.1) | 81 (75.0) |
1 Active group: treatment for 3 months with DeflaGyn® vaginal gel
2 Control group: 3 months "watchful waiting" without treatment
p16/Ki-67 after 6 months
| CINtec® PLUS | Patients n (%) | CINtec® PLUS status after 6 months | ||
|---|---|---|---|---|
| No data | Negative | Positive | ||
| DeflaGyn® vaginal gel – Active Group1 | ||||
| Negative | 30 (29.4) | 2 (6.7) | 28 (93.3) | 0 (0.0) |
| Positive | 72 (70.6) | 6 (8.3) | 60 (83.3) | 6 (8.3) |
| No data | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 102 (100.0) | 8 (7.8) | 88 (86.3) | 6 (5.9) |
| Watchful Waiting – Control Group2 | ||||
| Negative | 8 (7.5) | 0 (0.0) | 5 (62.5) | 3 (37.5) |
| Positive | 98 (91.6) | 1 (1.0) | 21 (21.4) | 76 (77.6) |
| No data | 1 (0.9) | 0 (0.0) | 0 (0.0) | 1 (100.0) |
| Total | 107 (100.0) | 1 (0.9) | 26 (24.3) | 80 (74.8) |
1 Active Group: 3 months treatment with DeflaGyn® vaginal gel + 3 months observation without treatment.
2 Control Group: 6 months Watchful Waiting (without treatment).
CINtec® PLUS Summary
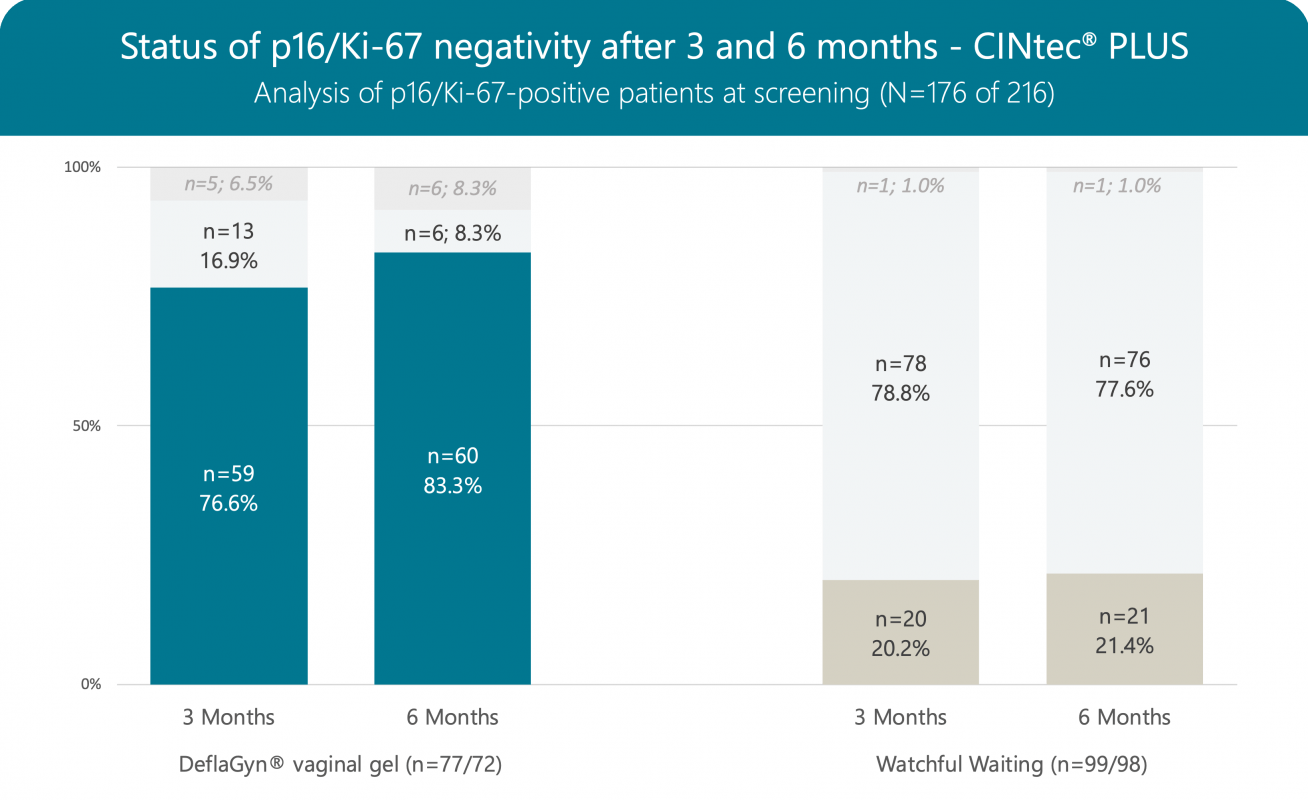
Clearance of p16/Ki-67 (positive at baseline and negative after 3 months) is 76.6% in patients treated with DeflaGyn® vaginal gel, compared to only 20.2% in patients without treatment.
Of 31 patients who were negative for CINtec® PLUS at baseline in the treated group, 30 patients (96.8%) remained negative after 3 months and none became positive (0.0%). Whereas in the untreated group 2 out of 8 patients (25.0%) became positive.
After 6 months (3 months treatment + 3 months observation without treatment), negativity for p16/Ki-67 increased from 76.6% to 83.3% in active group patients.
In patients who were negative for CINtec® PLUS at baseline, 28 out of 30 (93.3%) remained negative even after 6 months and none became positive. In the untreated group, 3 out of 8 patients (37.5%) became positive.
DeflaGyn® vaginal gel significantly impacts oncogenic progression. The considerable reduction in oncogenic risk factors (83.3% negative for p16/Ki-67) shows that DeflaGyn® vaginal gel may be an effective therapy for patients with HPV-caused cervical lesions.
DeflaGyn® vaginal gel – significant reduction of oncogenic risk factors
Physiological regeneration of epithelium
With the described three-dimensional mechanism, DeflaGyn® vaginal gel blocks reinfection and multiplication of HPV, thus improving conditions for spontaneous remission.
p16/Ki-67 negativity through HPV clearance
Helps interrupt the reinfection process through sustained elimination of HPV.
Application of DeflaGyn® vaginal gel
EFFECTIVE

SAFE

SIMPLE
To achieve the best possible effect, use DeflaGyn® vaginal gel once a day for a period of 3 x 28 days. It is recommended that application be done in the evening, before bedtime.
Patients who do not menstruate
DeflaGyn® vaginal gel should be used for a period of 3 x 28 days. After 28 days of treatment, stop use for 3 days. Then continue treatment for another 28 days and stop again for 3 days. Then continue with the third treatment cycle.
Patients who menstruate
Do not use DeflaGyn® vaginal gel during menstruation (approximately 3–5 days per cycle). It is not necessary to interrupt treatment for another 3 days, as menstruation itself counts as treatment break. You can start using the gel on any day of your cycle.
How to use the applicator
Please use the applicator in combination with DeflaGyn® vaginal gel to ensure correct application and dosage of the product.
Shake the bottle well before use. Extract 5 ml of gel by slowly pulling the applicator plunger until it reaches the 5 ml mark, and apply the gel deep into the vagina (until it reaches the cervix).

1 – Place tip
Place the DeflaGyn® vaginal gel bottle on a flat surface. Remove the bottle cap. Press the applicator tip onto the bottle opening.
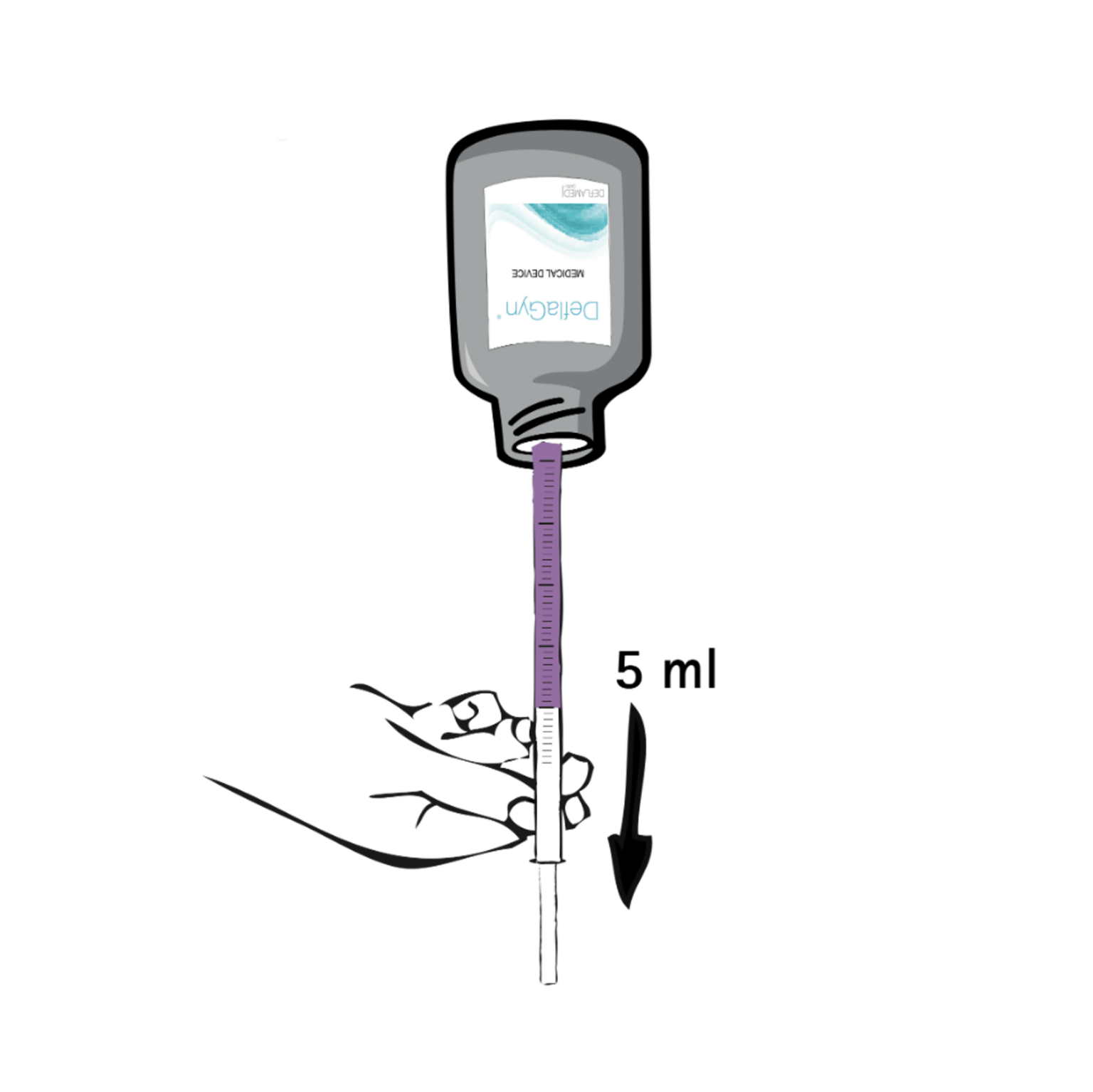
2 – Turn & pull
Turn the bottle upside down (180°) with the applicator in place. Slowly pull the plunger until it reaches the 5 ml mark. Remove the filled applicator from the bottle and place the bottle back on the table.
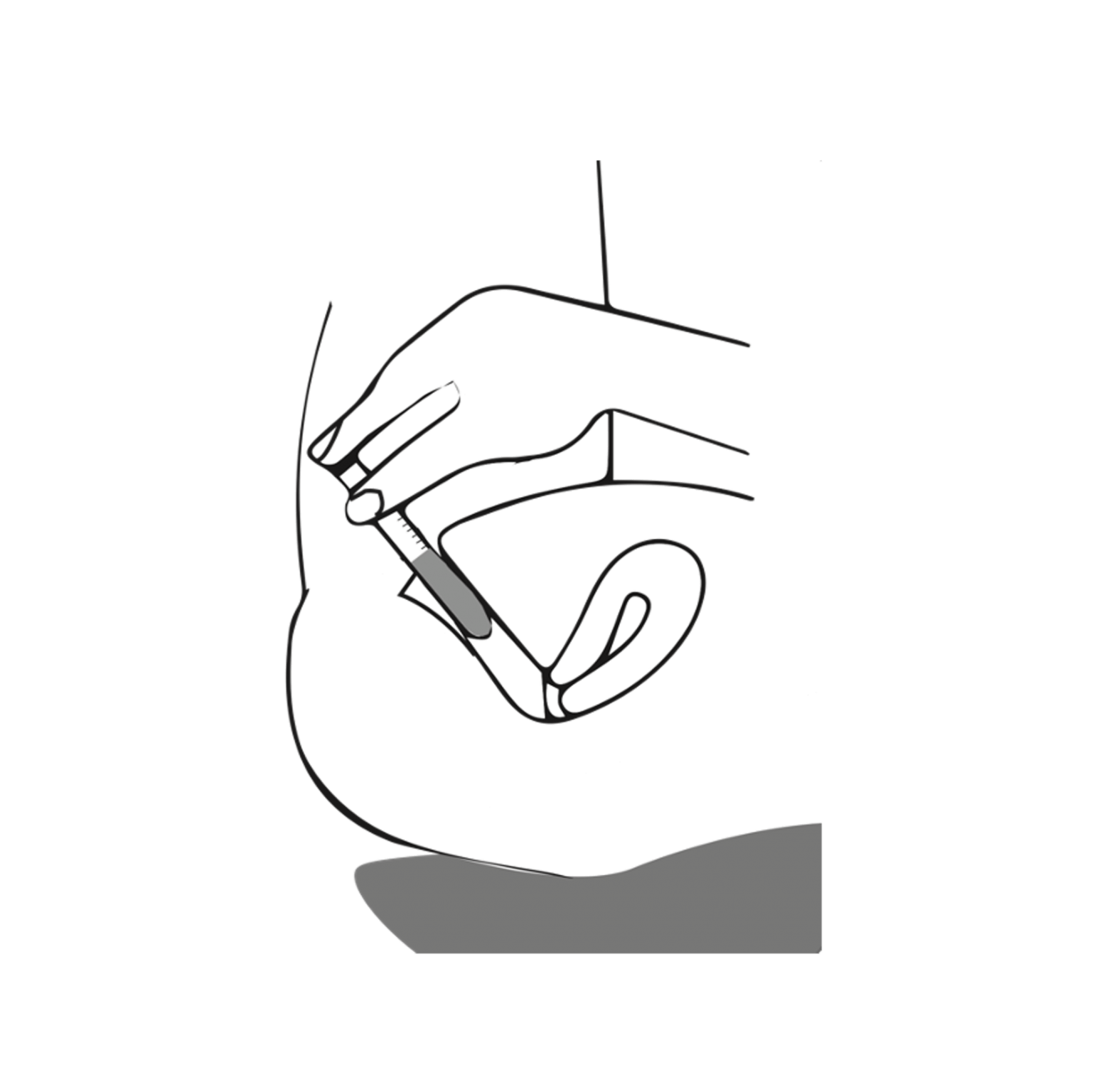
3 – Insert & press
Lie on your back with a pillow under your pelvis. Insert the applicator deep into the vagina until it touches the cervix. Once fully inserted, gently press the plunger to distribute the gel.
Preferably apply vaginal gel in the evening.
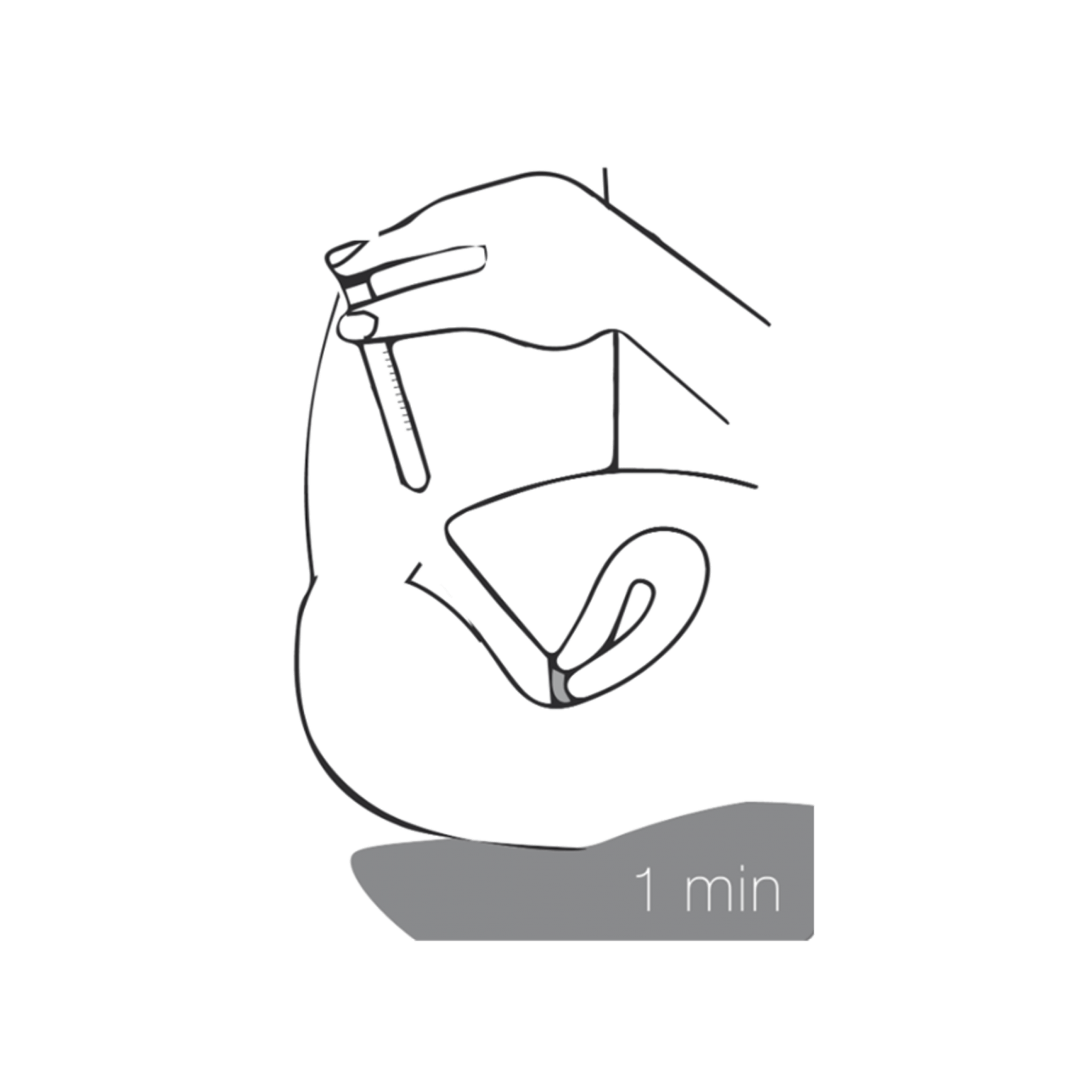
4 - Remove and stay lying
Remove the applicator from the vagina and remain in a lying position for about 1 minute. Make sure the pelvis remains elevated for proper distribution of vaginal gel to the cervix.
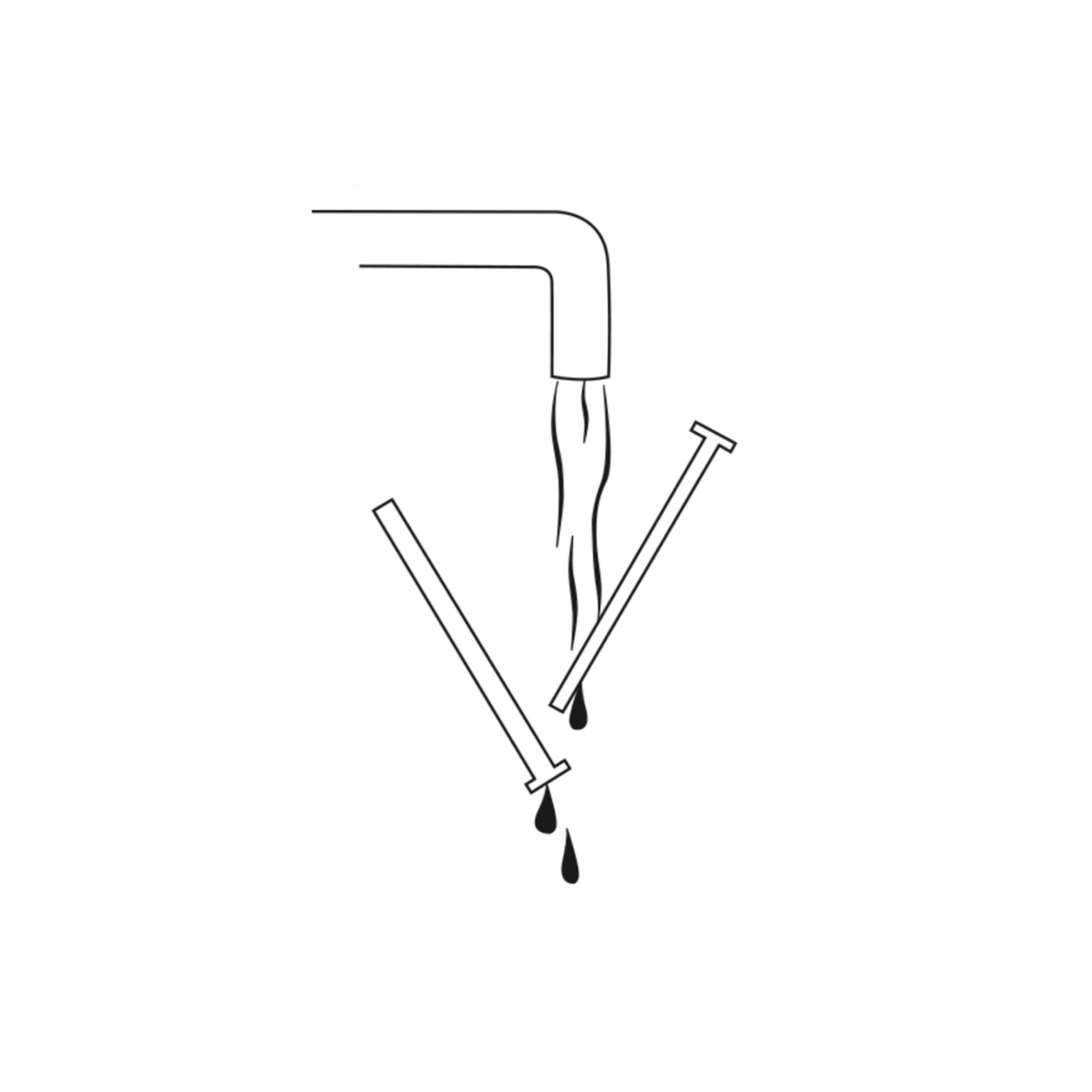
5 - Clean and dry
After use, clean the applicator well. Separate the tube and plunger and wash by hand with warm water (up to 50°C). Do not wash in dishwasher. Dry the parts and reassemble them.
The applicator can be used up to 28 times, if cleaned after each use.
Disposal
The used and cleaned applicator can be disposed of in household waste or plastic recycling, if possible.
Note
After application, some gel may leak out. This gel may have a reddish color and may stain your underwear.
However, stains disappear after washing. You can also use a sanitary pad.
Do not use the applicator if it is not cleaned well or if it is damaged. The applicator is dedicated only for DeflaGyn® vaginal gel.
Reusable applicators
- Easy to use and maintain
- Improved sliding mechanism
- Can be reused up to 28 times
- Durable, less packaging material
- Environmentally friendly – less plastic