Regular screening is prevention of cervical cancer
Screening is for healthy individuals without symptoms. Cervical cancer screening helps detect and treat cell changes in early stages.
Regular testing can save your life and is the most effective way to prevent cervical cancer.

Screening
Cervical screening is not a test for cancer, but a test to help prevent it. The main goal of screening is to identify precancerous lesions caused by HPV so they can be removed and prevent the development of invasive cancers.
A secondary goal is to detect cervical cancer at an early stage, when it can be successfully treated. Regular screening has been shown to significantly reduce the number of cases and deaths from cervical cancer.
Prevention
Cervical cancer can often be prevented through regular screening with Pap and HPV tests, which help detect precancerous changes and treat them. Additionally, the HPV vaccine is very effective in preventing new infections. It is recommended that girls be vaccinated against HPV.
The HPV vaccine offers the best protection when given at ages 9-12 years. It can prevent up to 90% of HPV-related cancer cases. Discuss with your doctor the most appropriate vaccination plan based on age, gender, and vaccine availability.
How is cervical cancer screening done?
Cervical cancer screening can be done at a clinic, community center, or at the doctor's office. It is usually performed during a pelvic examination.
Research shows that shame, cultural barriers, and fear are some of the reasons why women avoid screening.
The earlier HPV-induced changes are detected and treated, the better. This can save your life!
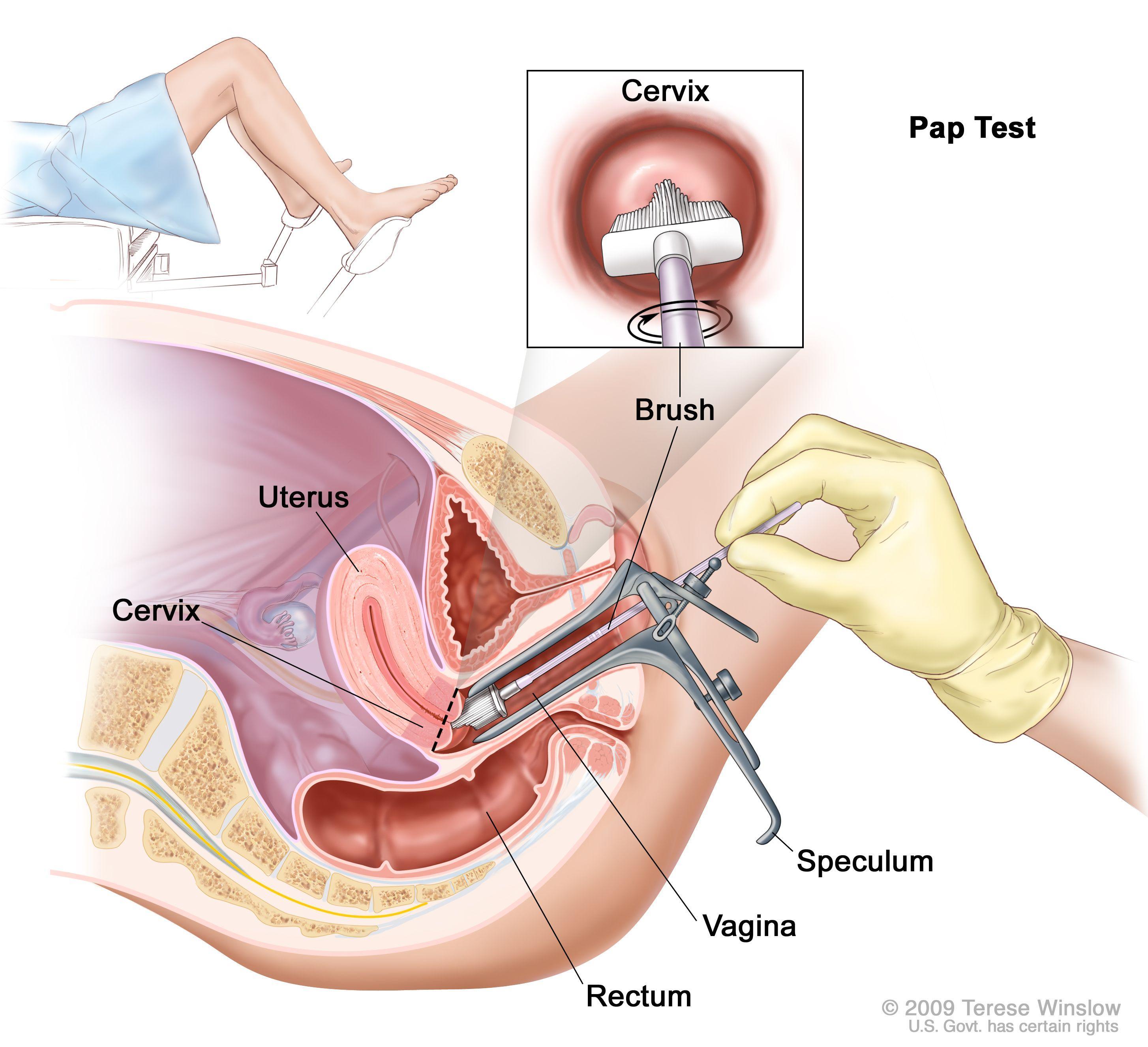
Typical Procedure
While a woman lies on an examination table, a healthcare professional inserts an instrument called a speculum into her vagina to widen it so that the upper part of the vagina and the cervix can be seen.
This procedure also allows the healthcare professional to take a sample of cervical cells. The cells are collected with a wooden or plastic spatula and/or a cervical brush and placed in a vial with liquid preservative.
In some countries, home testing is offered – a simple way for women to do the test themselves. These home kits provide an additional opportunity for women to stay up to date with screening.
Laboratory Testing
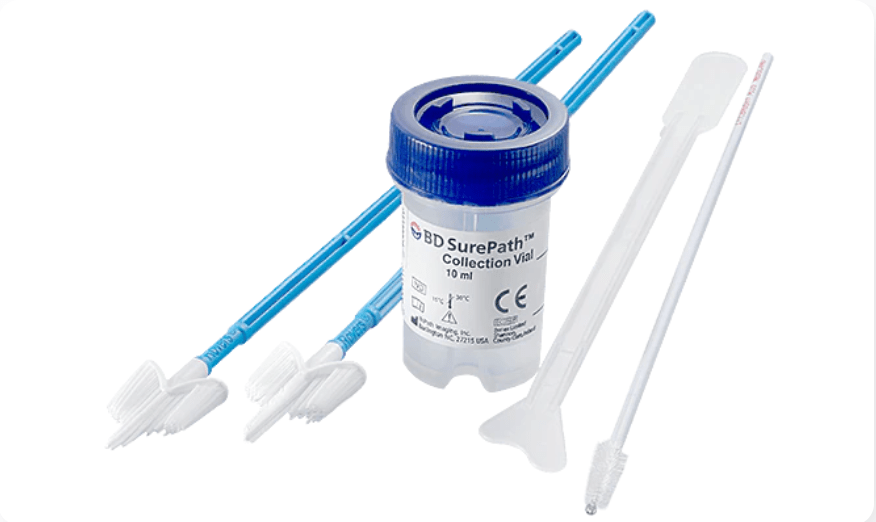
The sample is sent to a laboratory where cells are tested for high-risk types of HPV or analyzed under a microscope with a method called liquid-based Pap test. When the same sample is used for two tests (HPV + Pap), it is called "co-testing".
With the introduction of advanced computer-aided testing and new technology, the cytology laboratory is at the forefront of improvements to provide the best care for the patient.
The Bethesda System
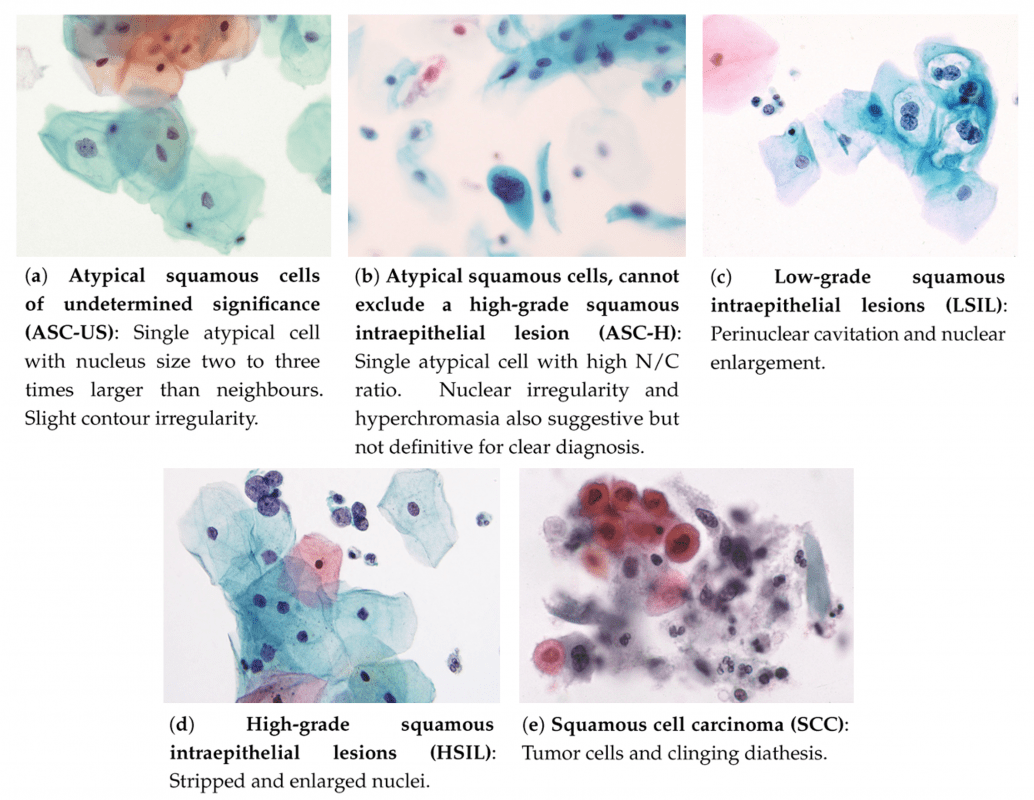
The Bethesda System separately analyzes squamous and glandular cell abnormalities. These two types meet in an area called the "transformation zone", which changes with age or after childbirth.
Squamous cell abnormalities are divided according to severity – from mildest to most serious.
Examples of atypical squamous cells in liquid-based cytology (LBC). Taken from: Nayar, R.; Wilbur, D., "The Bethesda System..."Squamous Cell Abnormalities
-
Atypical squamous cells (ASC) are the most common finding in Pap tests. They are divided into two groups: ASC-US and ASC-H:
- ASC-US: Atypical cells of undetermined significance – not normally appearing, but the reason may be an HPV infection or other factors.
- ASC-H: Cells that do not exclude the presence of high-grade lesion. Higher risk for cancer development.
- LSIL: Low-grade lesions, usually associated with HPV infection. Often clear on their own in young women.
- HSIL: High-grade lesions – more likely to progress to cancer if untreated.
- CIS (karcinoma in situ): Cells that look cancerous but have not spread beyond the cervical surface.
- Squamous carcinoma: An advanced form of cancer – when abnormal cells have penetrated deeper into tissues or neighboring organs.
Glandular Cell Abnormalities
Atypical glandular cells (AGC) usually come from the glandular epithelium of the endocervix or endometrium. These are less common than squamous cells.
These abnormalities include abnormal changes in the glandular tissues of the cervix. The Bethesda System divides them into these categories:
- Atypical glandular cells (AGC): Cells do not look normal, but doctors are not sure of their meaning.
- Adenokarcinoma in situ (AIS): Very abnormal cells, but which have not spread beyond the glandular tissue.
- Adenokarcinoma: May include cancer not only in the endocervical canal but also in the uterus, endometrium, or other areas.
Munich Nomenclature
Different classification systems have been used for analyzing Pap samples at an international level. Germany has used the "Munich" nomenclature since 1975 as a modification of the Papanicolaou classification.
This modification has been adapted to meet international standards for descriptive classifications. In 2015, it was officially established as a system in Germany.
With "Munich III" subgroups were created for classifying suspicious changes that do not clearly match existing criteria. The system further differentiated between squamous, epithelial and glandular cells.
The goal is comparison with systems such as Bethesda, to enable international analysis and more accurate comparisons.
Screening Methods
For many years, the Pap test was the only method for cervical cancer screening. Its use helped reduce incidence and deaths in countries where it is used regularly.
Today there are three approaches: HPV test (for presence of high-risk types), Pap test, and combined HPV/Pap test (co-test), which uses the same sample to check both.
HPV Test and Pap Test
The HPV test and Pap test are two different screening methods. They are used to identify changes in cervical cells before symptoms appear.
Women need regular screenings, even if they feel well. This helps in early detection and proper treatment to stay healthy.
- The HPV test checks for infections with high-risk HPV types.
- The Pap test (or cytology) collects cells to see changes that may lead to cancer or detect cancerous cells.
- The combined Pap/HPV test is used to check both: HPV infection and cell changes.
CINtec® PLUS
CINtec® PLUS Cytology is a specialized test for cervical cancer screening. It uses two biomarkers (p16 and Ki-67) to accurately detect transformative HPV infections.
CINtec® PLUS helps doctors identify patients with suspicious results from Pap and HPV, to recommend better follow-up through colposcopy.
- The test detects HPV infections that have potential to cause cancer and avoids incorrect interpretations.
- Shows high clinical accuracy in different cervical cancer screening scenarios.
- Dual staining p16/Ki-67 may significantly reduce the number of women sent for unnecessary colposcopies.
Cervical Erosions
Cervical erosion, also known as cervical eversion, ectropion or cervical ectopy, is a common condition in women of reproductive age and usually poses no risk. It is not an indicator of any other health problem, such as cervical cancer. If symptoms concern you, there are treatments you can discuss with your doctor.
Cervical ectropion occurs when cells from the inside of the cervix grow on the surface. These cells are more sensitive and redder, so they may cause symptoms such as bleeding or discharge in some women.